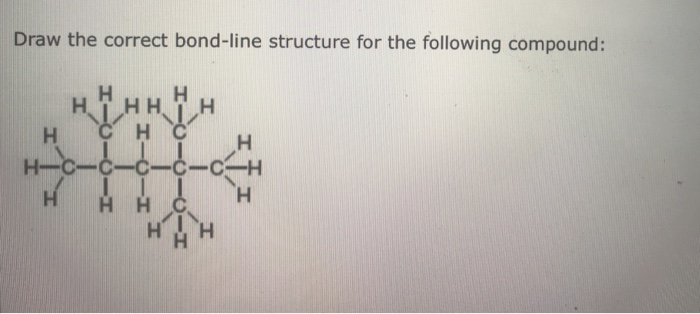
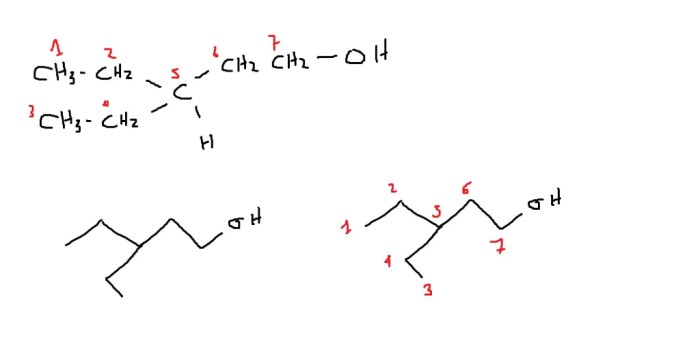
Draw the correct bond-line structure for the following compound – Drawing the correct bond-line structure for a given compound is a fundamental skill in organic chemistry. Bond-line structures are simplified representations of molecules that convey essential structural information in a concise and easy-to-understand format. This guide provides a comprehensive overview of the principles and techniques involved in drawing accurate bond-line structures, empowering chemists to effectively communicate molecular structures and facilitate deeper understanding of chemical concepts.
Understanding bond-line structures is crucial for visualizing molecular structures, identifying functional groups, and predicting molecular properties. By mastering the art of drawing bond-line structures, chemists gain a valuable tool for effectively conveying complex chemical information and advancing their research endeavors.
Bond-Line Structures: A Guide to Drawing Accurate Representations: Draw The Correct Bond-line Structure For The Following Compound
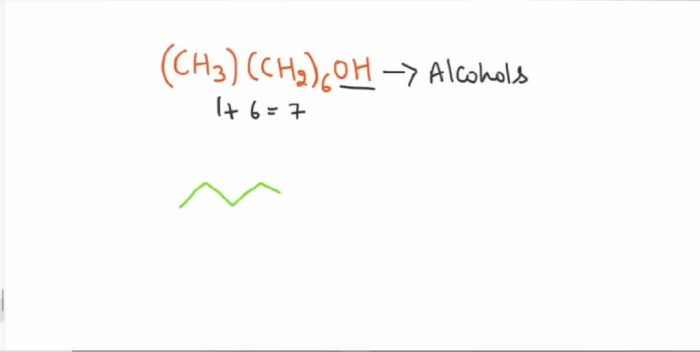
Bond-line structures are simplified representations of organic molecules that convey the connectivity and arrangement of atoms and bonds. They are essential for visualizing and understanding the structure and properties of organic compounds.
General Guidelines for Drawing Bond-Line Structures, Draw the correct bond-line structure for the following compound
The following rules govern the drawing of bond-line structures:
- Carbon atoms are represented by intersections of lines or angles.
- Hydrogen atoms attached to carbon atoms are not shown.
- Heteroatoms (atoms other than carbon and hydrogen) are represented by their chemical symbols.
- Single bonds are represented by single lines, double bonds by double lines, and triple bonds by triple lines.
Identifying Functional Groups and Skeletal Structures
Functional groups are specific arrangements of atoms that impart characteristic properties to organic compounds. Skeletal structures are the carbon frameworks of molecules, excluding hydrogen atoms.
Common functional groups include alcohols (OH), alkenes (C=C), and ketones (C=O). Their bond-line representations are:
- Alcohol: -OH
- Alkene: C=C
- Ketone: C=O
Representing Branching and Multiple Bonds
Branching in molecules is represented by forking lines. Multiple bonds between carbon atoms are shown as double or triple lines connecting the carbon atoms.
For example, the bond-line structure of 2-butene, a branched alkene, is:
CH3
|
CH=CH-CH3
Advanced Techniques for Complex Structures
For complex molecules, advanced techniques are used to represent cyclic structures, resonance structures, and stereochemistry.
Cyclic structures are represented by connecting the carbon atoms in a ring. Resonance structures are represented by showing the different possible electron distributions within a molecule.
Stereochemistry is represented by using wedges and dashes to indicate the spatial orientation of atoms.
Examples and Practice Exercises
Examples of bond-line structures include:
- Methane: CH4
- Ethanol: CH3CH2OH
- Benzene: C6H6
Practice exercises for drawing bond-line structures are available in textbooks and online resources.
Key Questions Answered
What are the basic rules for drawing bond-line structures?
The basic rules for drawing bond-line structures include representing carbon atoms as corners or intersections of lines, hydrogen atoms as lines attached to carbon atoms, and other atoms as symbols placed at the ends of lines.
How do you represent branching and multiple bonds in bond-line structures?
Branching is represented by lines extending from the main carbon chain, and multiple bonds are indicated by additional lines connecting the bonded atoms.
What are some advanced techniques for drawing bond-line structures of complex molecules?
Advanced techniques include using wedges and dashes to represent stereochemistry, and using resonance structures to represent multiple possible electron configurations.