Embarking on an exploration of the fundamental building blocks of our universe, we present the States of Matter Basics Answer Key. This comprehensive guide unravels the intricacies of matter’s three primary states—solid, liquid, and gas—illuminating their unique properties and intermolecular dynamics.
Delving into the realm of phase transitions, we decipher the processes that govern matter’s transformation from one state to another. Intermolecular forces, the invisible bonds that shape matter’s behavior, are meticulously examined, revealing their profound influence on a substance’s characteristics.
States of Matter Basics
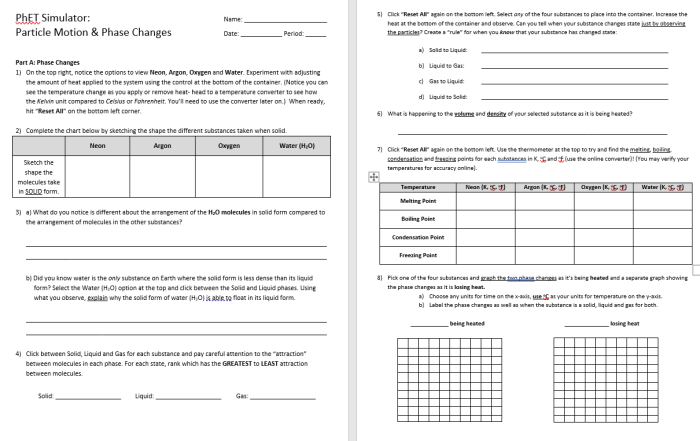
Matter exists in three fundamental states: solid, liquid, and gas. Each state exhibits unique properties and characteristics that distinguish it from the others.
Solid
- Definite shape and volume
- Particles are closely packed and arranged in a regular pattern
- Low kinetic energy
- Examples: ice, metal, wood
Liquid
- Definite volume but no definite shape
- Particles are closely packed but can move more freely
- Higher kinetic energy than solids
- Examples: water, oil, mercury
Gas
- No definite shape or volume
- Particles are widely spaced and move rapidly
- Highest kinetic energy of the three states
- Examples: air, helium, hydrogen
Phase Transitions
Phase transitions occur when matter changes from one state to another. These transitions involve changes in energy and molecular arrangement.
Types of Phase Transitions, States of matter basics answer key
- Melting (solid to liquid)
- Freezing (liquid to solid)
- Vaporization (liquid to gas)
- Condensation (gas to liquid)
- Sublimation (solid to gas)
- Deposition (gas to solid)
Intermolecular Forces: States Of Matter Basics Answer Key
Intermolecular forces are attractive forces between molecules. These forces influence the properties of matter and determine the state of matter at a given temperature and pressure.
Types of Intermolecular Forces
- Hydrogen bonding
- Dipole-dipole interactions
- London dispersion forces
The strength of intermolecular forces affects the melting point, boiling point, and other physical properties of matter.
Kinetic Molecular Theory
Kinetic molecular theory explains the behavior of gases by considering the motion and interactions of their molecules.
Postulates of Kinetic Molecular Theory
- Gas molecules are in constant random motion.
- The average kinetic energy of gas molecules is proportional to the absolute temperature.
- Gas molecules collide with each other and with the walls of their container.
- These collisions are elastic, meaning that no energy is lost.
Kinetic molecular theory provides a framework for understanding the behavior of gases, including their pressure, volume, and temperature relationships.
Applications of States of Matter
The states of matter have numerous applications in everyday life and various technologies.
Applications in Everyday Life
- Solid: building materials, furniture, tools
- Liquid: water, beverages, cleaning solutions
- Gas: air, fuel, anesthetics
Applications in Technologies
- Solid: semiconductors, superconductors
- Liquid: hydraulic systems, heat transfer fluids
- Gas: lasers, refrigeration systems
FAQs
What are the defining characteristics of a solid?
Solids possess a fixed shape and volume due to the strong intermolecular forces that hold their particles tightly packed in a rigid structure.
How does temperature affect the state of matter?
Increasing temperature generally leads to a transition from solid to liquid to gas, as the increased kinetic energy of particles overcomes the intermolecular forces holding them together.